3D Microprinted Bones for an Actuated Joint-on-Chip Model
PIs:
Dr. Xiao-Hua Qin, Micro-Tissue Engineering and Biomaterials Team, Laboratory for Bone Biomechanics, ETH Zurich
Prof. Ralph Müller, Laboratory for Bone Biomechanics, ETH Zurich
Fellow:
Margherita Bernero, Micro-Tissue Engineering and Biomaterials Team, Laboratory for Bone Biomechanics, ETH Zurich
Collaborators:
Xiaoyu Zhao and Prof. Marcy Zenobi-Wong, Tissue Engineering and Biofabrication, ETH Zurich
Ali Kerem Kalkan and Prof. Ori Bar-Nur, Laboratory of Regenerative and Movement Biology, ETH Zurich
Rodrigo Castillo-Acuña and Prof. Dennis Kochmann, Mechanics and Materials Laboratory, ETH Zurich
Background
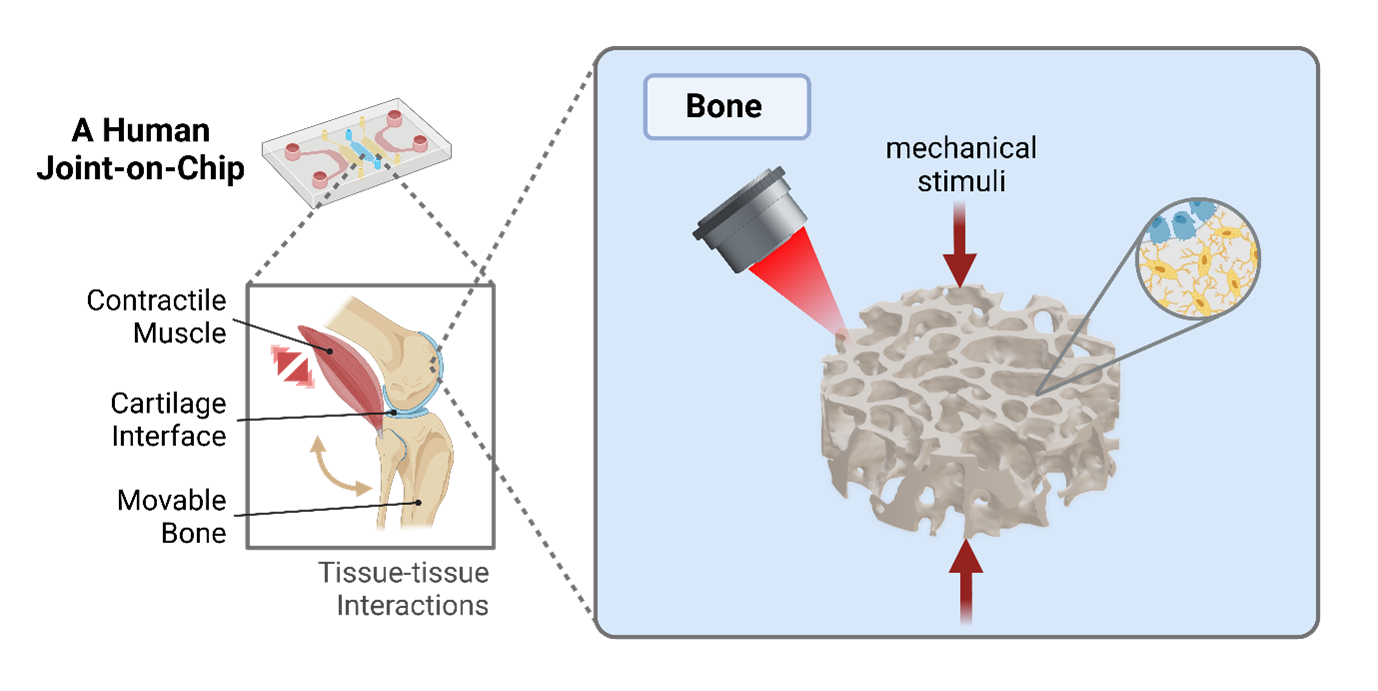
Osteoarthritis (OA), a complex disease affecting the entire joint, has a huge socioeconomic impact in our aging population. Despite its high prevalence, the mechanisms underlying the disease remain elusive which is hindering the development of targeted OA drugs. In order to establish a clinically relevant, resource-saving and high-throughput platform to advance our understanding of joint physiology and pathology, we propose a microphysiological, actuated human joint-on-chip.
One component of this collaborative project will be the engineering of a functional bone tissue construct in vitro. Bone development, structure and remodeling in vivo are tightly regulated by mechanical stimuli. However, in vitro models that can recapitulate both bone microarchitecture and maturation under physiological loads are missing.
Goal
The overall goal of this project as part of the ALIVE Zurich Joint stream is to develop a 3D microprinted human bone model that is compatible with mechanical loading and multi-tissue-on-chip integration. As part of a movable in vitro joint model, the proposed bone tissue component will contain an in vivo-like cellular composition and microarchitecture and will mature under dynamic mechanical stimulation.
Methodology
We use high-resolution biofabrication techniques to produce architected bone-like tissue constructs. Firstly, we develop advanced photoresponsive hydrogels that allow 3D microprinting of permissive hydrogel environments for in vitro 3D osteogenic differentiation. Then, we will develop a custom chip designed for physiological mechanical actuation of the constructs to accelerate functional tissue maturation. To maximize the osteogenic potential of our system, the effect of different hydrogel compositions, construct architectures and loading parameters will be evaluated by state-of-the-art bioimaging and biomolecular assays. Eventually, the integration with other joint tissue components (e.g., cartilage and muscle) will lead to a functional in vitro model of the human joint that can be used for the study of cell mechanobiology and as a clinically relevant model for the development of novel pharmaceutical therapies.