Multiscale Biohybrid Tissues for Robotics Driven by Applications in Health (BHR)
Core members:
Edoardo Mazza, Jess Snedeker, Lucio Isa, Robert Katzschmann* & Simone Schürle
*stream leader
Motivation & relevance
Soft robots often resemble living organisms, are inspired by their forms, kinetics and functions and may be constructed as biohybrid systems incorporating living materials. Their innate softness allows them to naturally interface or even integrate with humans. As such, they bear tremendous potential becoming key elements in future healthcare, as robots for elderly care, assisting with rehabilitation therapy or maybe one day as seemingless integrated prosthetics. Advancements and insights gained from the development of living materials for soft robotics will assist the evolution of current diagnostics, therapeutics and treatment technologies. These advances will address the core challenges of our aging society and provide a new class of medical systems for future healthcare.
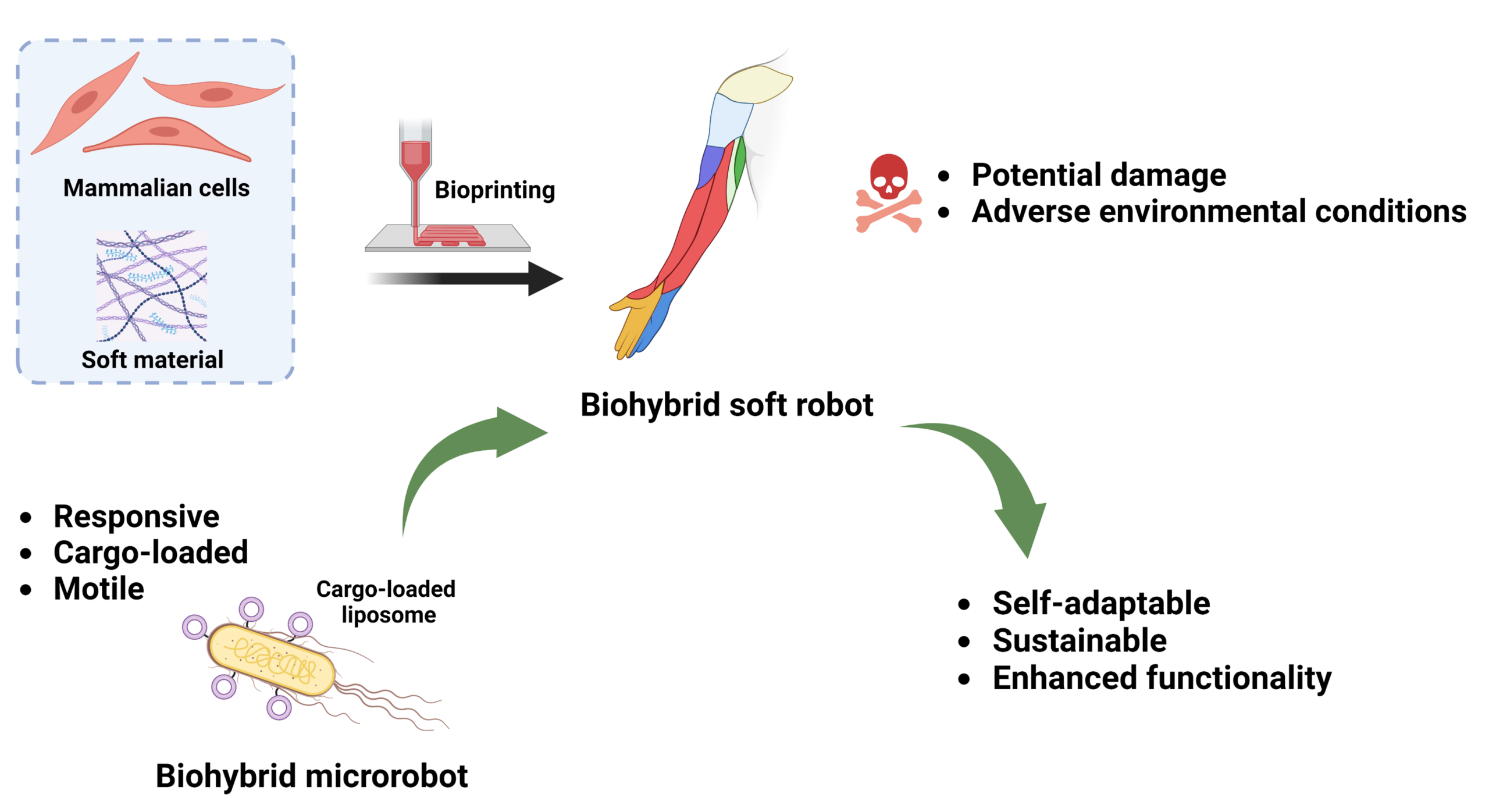
To realise this transformation, these soft medical robots should be self-sustained, made from sustainable material, respond to environmental cues and act autonomously. Components made from mammalian cells, such as myocytes, can proliferate and grow, and the electronics can be replaced with microorganisms or other cells as sustainable sensing and signalling nodes. For example, the quorum sensing capability of certain bacteria species can be converted into binary signals and used for the communication of robots when they incorporate into robots' bodies during the fabrication process. Furthermore, the responses of organisms to the changes in their surrounding environments, (such as temperature, mechanical and chemical stimuli), can serve to develop living sensors that can be used as robotic components. The ability of organisms to convert chemical energy into electrical or mechanical ones can be used to create robotic actuators or bio-power generators for decreasing greenhouse gas emissions and pollution. Overall, living materials as responsibly used natural resources provide great opportunities to realise sustainable robotic components as a basis for next-generation medical soft robots.
Key objectives
We aim to formulate 3D-printable living materials using muscle and nerve cells and to embed them in a controllable assessment environment. Our main objective is to create capable soft robotic actuators that mimic the smooth and energy-efficient motion of animals while operating in a setting outside of a host body. To achieve this objective, it is crucial to scale up current biological constructs from micro- to decimeter scale while keeping them viable and functional. The realisation of such robotic bio-actuators requires an interdisciplinary research effort that brings together scientists from tissue engineering, materials science, mechanobiology, control engineering and robotics. Upon reaching this objective, we aim to create a bridge between these actuators and unanimated robot structures by fabricating tendon-like structures that match in compliance. Control theory will serve as a central element to orchestrate function at the micro and macroscale and combine the two. For this purpose, we envision to deterministically interact with cells. Concretely, we seek to receive cell response to specific stimuli in real-time and design closed-loop control algorithms to achieve certain functions. We anticipate new levels of robotic performance with a higher force outcome. This effort necessitates the formulation of different living materials with varying mechanical properties and are compatible with multimaterial printing technologies. We also require control techniques on the cellular level to coordinate and make these biohybrid constructs viable. Furthermore, we anticipate controllable microagents deployed within the biohybrid actuators.
Rationale & methods to be employed
As a fundamental concept, we plan to use multimaterial bio-printers to achieve our key objectives. For the scaling up of biological constructs, we initially examine the hierarchical fabrication of muscle bundles separated with several perfusion channels that can supply oxygen and nutrients to the nearest living entity throughout the whole construct. Next, we aim to fabricate more complex biological constructs composed of vessel-like channels using endothelial cells. Finally, the actuation of the muscle bundles will be controlled by integrating nerve cells into the constructs. The crucial points at this final step are the formation of functional neuromuscular junctions between the nerves and muscles and the efficient nutrient and oxygen supplies to the whole living entities found within the constructs. To connect the bioconstructs with unanimated robot components, we aim to formulate 3D printable materials whose mechanical properties can be tuned during the printing process via extending the polymerisation reaction period or applying chemicals that can affect the stiffness of the printed parts. Integration of engineered microorganisms or synthetic materials will function as local microscale sensing and signalling nodes and provide a sub-layer of intelligence. These microorganisms can be externally controlled or deterministically respond to certain environmental cues. Genetic circuits will be integrated on a single cell level for specific function and swarm control principles will be employed for collective actuation.
Impact, expected output & added value of project
This project has a broad impact area that can improve our way of living and life quality by enabling the development of the next-generation of soft robots and the fabrication of large-scale implantable tissue constructs. On that route, knowledge will be gained that will serve for the advancement of current diagnostic and therapeutic technologies based on living organisms. In 5 years, we are convinced to be able to realise living soft robots that can perform locomotion and object manipulation outside of the laboratories. In 10 years, we envision multifunctional large-scale constructs with varying biological and mechanical properties that can be used as simple animal models and maybe even as implants for tissue regeneration.
Projects
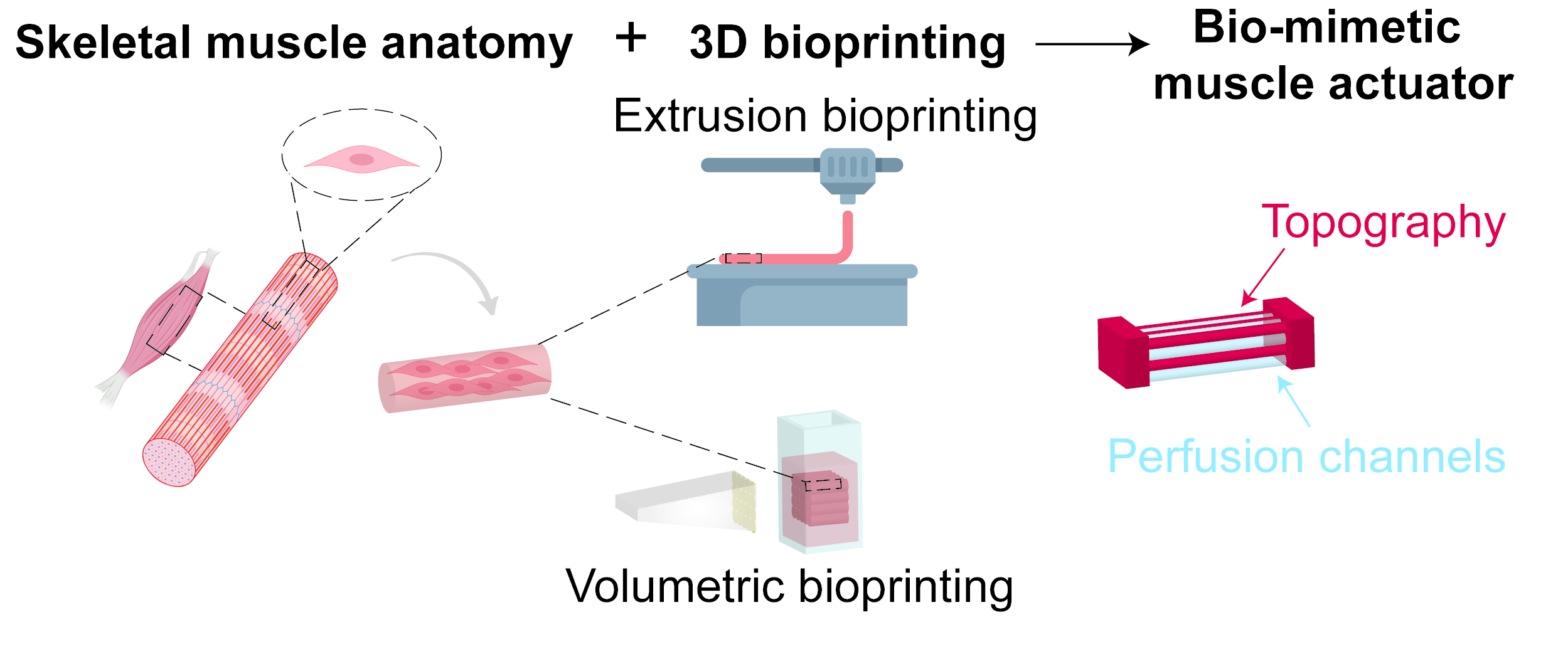
Engineering a cm-scale contractile 3D skeletal muscle actuators for biohybrid robots
Biological muscles carry the promise of being energy efficient and soft actuators for next generation robots. However, we are currently limited in our ability to engineer contractile skeletal muscle constructs past the mm-scale, mainly due to the challenges of nutrient perfusion and lack of ability to recapitulate the necessary biological and mechanical features of the native muscle extracellular matrix using available biofabrication techniques. In this project, we aim to innovate on current biofabrication methods in order to engineer a cm-scale skeletal muscle tissue for actuating a bio-hybrid robot, capable of a higher force generation than current state-of-the-art skeletal muscle actuators.
Read more
Biohybrid microrobots for damage detection and repair in large scale biohybrid systems
Soft biohybrid robot systems consist of live cells prone to damage. We aim to engineer a subclass of biohybrid microrobots based on motile live microorganism that surveil the robotic construct to identify potential damage and aid with repair.
Biohybrid Bacterial Systems
Harnessing the power of motile bacteria may serve as a compelling strategy for active transport. We aim to create biohybrid microrobots in a highly precise and reproducible manner exploiting a novel directed assembly technique based on capillary forces.
Characterization and Modeling of Biomaterials for applications in Biohybrid Robotics
Recent advancements in the field of biohybrid robotics, such as optogenetic muscle bio actuators, self-propelling biohybrid swimmers, and 3D bioprinting of active materials, have demonstrated promising applications. However, despite these exciting possibilities, a distinct gap remains in the literature concerning the characterization and computational modelling of biomaterials, which are crucial for robot design and fabrication. This project seeks to address these gaps, and understand the underlying biological and mechanical processes to reliably create biomaterial systems that behave in a predictable and desirable manner.
